Engineered Dendritic Cells Boost Cancer Immunotherapy
Engineering dendritic cells to better identify cancer cells and trigger precise immune responses may boost immunotherapy.
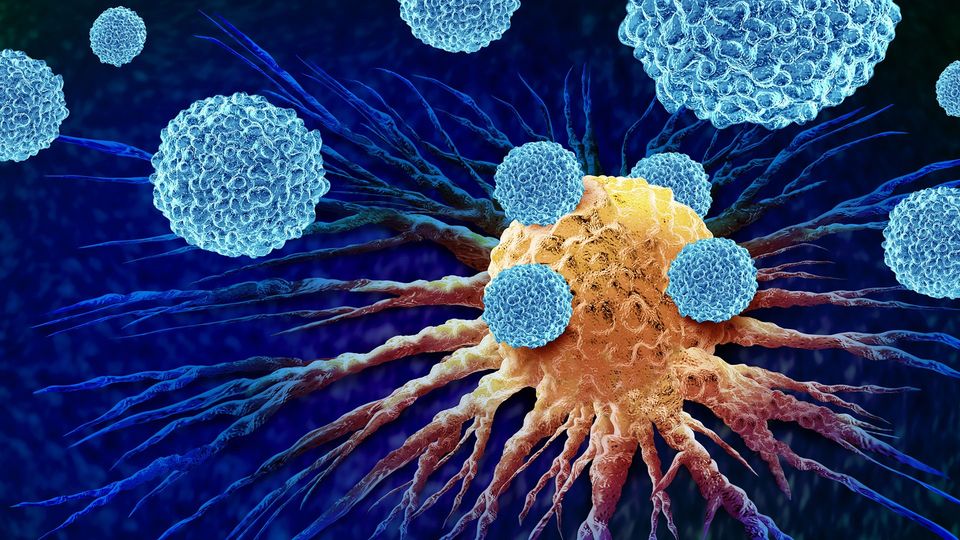
Cancer immunotherapy is a strategy that turns the patient’s own immune cells into a “search-and-destroy” force that attacks the tumor’s cells. The “search” immune cells are the dendritic cells, which collect and present identifying parts of the cancer cells (antigens) to the “destroy” part (T cells), the immune system's killer cells.
The problem is that many tumors “learn” how to evade detection by the patient's dendritic cells. Clinicians address this by collecting dendritic cells from the patient’s blood, loading them in the laboratory with tumor material – antigens that train dendritic cells to better identify the tumor – and then injecting them back into the patient.
However, tumors contain many more antigens than those supplied to the dendritic cells in the lab. Also, lab‑grown dendritic cells often lack some key activation molecules that are needed to fully engage T cells.
A solution can be found in extracellular vesicles (EVs): tiny packets released by cancer cells that carry proteins and other molecules that can help dendritic cells identify the tumor. If dendritic cells could pick up tumor EVs inside the patient’s body, they could trigger more precise and effective immune responses against the tumor.
A preclinical study
That is what scientists led by Professor Michele De Palma at EPFL have done, developing two bioengineering approaches that exploit cancer EVs and better train dendritic cells to identify cancer cells without the need to load them with tumor material outside the patient’s body.
The first approach, published in Nature Communications, uses a receptor called EVIR (“EV-internalizing receptor”) to help dendritic‑cell progenitors (immature dendritic cells) take up tumor-derived EVs and more effectively present their antigens to T cells.
De Palma’s lab first developed EVIR in 2018, but this new paper brings the research into a preclinical study. The EVIR-engineered dendritic cells elicited robust immune responses and inhibited the growth of experimental melanomas that are otherwise resistant to mainstream immunotherapy.
The second approach, published in Science Translational Medicine, improves the performance of EVIR so that dendritic cells not only internalize the tumor’s EVs and present its identifying antigens to T cells, but also spontaneously produce additional molecules that can further stimulate them into action. The result is iCAR, (“instructive chimeric antigen receptor”), which enables dendritic cells to better activate T cells against the tumor.
Birth of a start-up
The studies were conducted by two doctoral students, Ali Ghasemi and Yahya Mohammadzadeh. Together, they outline a path toward engineered dendritic cells that are programmed to acquire and present relevant tumor antigens directly in the body, instead of relying on limited tumor material provided to them in the lab.
“Our goal is to relaunch the clinical potential of dendritic-cell-based therapies through engineering them for improved performance in vivo – enhanced antigen uptake coupled to cell activation, without the need for antigen exposure ex vivo,” says Michele De Palma. “To sustain and expand these efforts, I encouraged the founding of EVIR Therapeutics, a biotechnology start-up created to recruit investment and strategic partnerships for bridging the gap between innovation and clinical testing.”
References: Mohammadzadeh Y, Gligorovski V, Egorova O, et al. Coordinate tumor-antigen uptake and dendritic cell activation by chimeric antigen receptors. Science Translational Medicine. 2025;17(829):eadq4060. doi:10.1126/scitranslmed.adq4060
Ghasemi A, Martinez-Usatorre A, Liu Y, et al. Dendritic cell progenitors engineered to express extracellular-vesicle–internalizing receptors enhance cancer immunotherapy in mouse models. Nat Commun. 2025;16(1):9148. doi:10.1038/s41467-025-64172-w
This article has been republished from the following materials. Note: material may have been edited for length and content. For further information, please contact the cited source. Our press release publishing policy can be accessed here.