“There’s No Reason To Accept Menopause”: Dr. Sherman Silber Breaks Fertility Myths
Fertility pioneer Dr. Sherman Silber reflects on innovation, ethics and extending fertility in reproductive medicine.
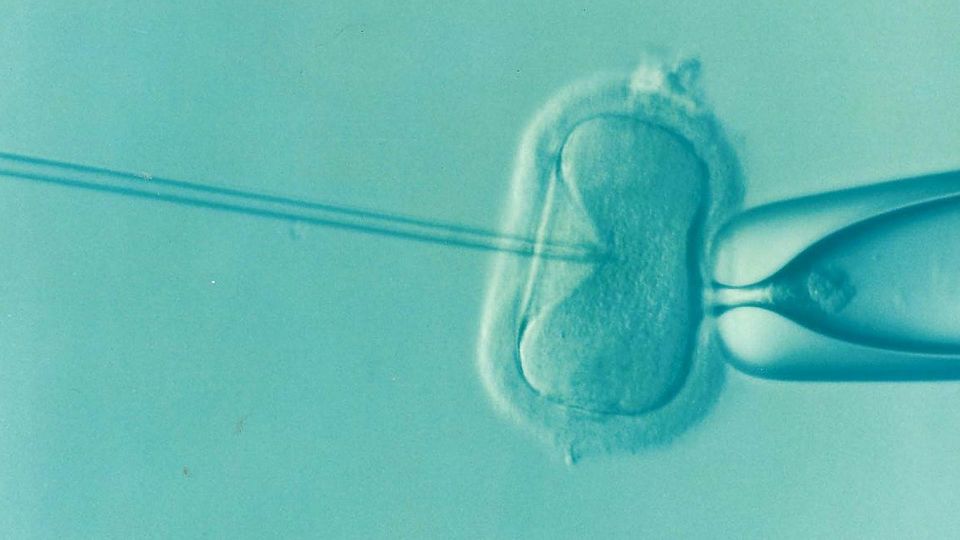
For more than five decades, Dr. Sherman J. Silber has been a driving force in reproductive medicine – consistently at the precipice of what many considered possible.
From introducing the world’s first microsurgical vasectomy reversal in 1975 to pioneering techniques in sperm retrieval, intracytoplasmic sperm injection (ICSI), ovary transplantation and the use of vitrification for egg and embryo freezing, his influence spans the breadth of fertility science.
Technology Networks spoke with Silber about the challenges and ethical considerations of introducing controversial fertility procedures, the resistance he has faced from both colleagues and the wider scientific community and the strategies he uses to ensure patient safety while pushing the boundaries of reproductive medicine.
He also discusses the intersection of fertility treatments and the science of aging, including emerging techniques to preserve ovarian function and extend female fertility, and shares his perspective on how curiosity, risk-taking and careful oversight drive innovation in this rapidly evolving field.
Even with a résumé full of medical firsts, Silber remains focused on one central mission: enabling families to have children safely and ethically.
Many of the techniques you pioneered were once considered experimental. How did you approach balancing risk, innovation and ethical responsibility when introducing these procedures?
Every new paradigm shift might be called unethical at first, just because it’s new and different. The key is to answer the question, “What is the risk of doing something new?” You need to track that risk, so you don’t go off the deep end.
Back in 1975, I was on the cover of The New York Times for my breakthrough development of microsurgical vasectomy reversal. At the time, I was attacked from all sides by doctors saying that vasectomies were meant to be permanent, so reversing them is immoral.
The way ethics work is this: Is it a serious risk to the patient? Is it a risk to society? How big is the risk when compared to the benefits? Nothing about reversing vasectomies, for example, is a serious risk to the patient or society, so it is ethical. It’s very simple.
It’s also important, of course, to study new techniques very carefully. For example, when developing ICSI, we worked with a team of geneticists to study every baby and follow those children closely. We also follow established health safety standards and make sure that every patient involved in an experimental procedure makes the decisions himself or herself, with proper informed consent. Moving carefully helps ensure that benefits outweigh risks.
When scientists come up with something new that differs from other scientists’ or physicians’ expertise, those people tend to feel threatened.
Like the huge negative outcry I got about microsurgical vasectomy reversal, I got fierce pushback for developing ICSI and testicular sperm extraction, which are now standard practices in in vitro fertilization (IVF) clinics all over the world. The same thing happened when I introduced vitrification to the United States to replace slow freezing for cryopreservation of eggs and embryos.
Vitrification
Vitrification is a rapid freezing process that turns cells, such as eggs or embryos, into a glass-like state without forming ice crystals, protecting them from damage.
Vitrification is 100% safe for eggs and embryos, and we don’t have to worry about the process destroying them because there is no formation of ice crystals whatsoever. But when we introduced it, we were attacked by many scientists who believed in slow freezing and had invested in slow freezing machines. Today, vitrification is standard practice.
People will always be shocked by new ideas that challenge the status quo. Some will get jealous and antagonistic, and some might try to undermine you to take the recognition themselves.
That happened to my late friend Dr. Kary Mullis, who won a Nobel Prize for developing the polymerase chain reaction method that led to genome sequencing. He couldn’t find a publisher for his research at first, but he didn’t give up until he found one because he knew others were racing to replicate his work and take credit for it.
We are experiencing that right now with our mini-IVF protocol. But the fact is, it works better than traditional IVF in a lot of cases. We just had a patient who is over 40 years old and has tried IVF elsewhere. They used too much gonadotropin and got terrible eggs and aneuploid embryos that couldn’t be used. She came to me for mini-IVF, and she got 42 eggs, got pregnant right away and now has a happy, healthy baby.
Cases like hers – and we have many – reconfirm that we need to keep pushing this approach forward. It requires change, and change is hard for people at first. But it’s the right thing to do for patients.
What we know is that women are born with all the eggs, or oocytes, they are ever going to have. If it weren’t for the fact that they were arrested, or suppressed, by the stiff outer cortex of the ovary while still in the fetal stage, they would all go into meiosis by the time a female is born.
Meiosis
Meiosis is a type of cell division that produces eggs or sperm, each with half the number of chromosomes of the original cell.
The outer cortex of the ovary is the toughest tissue in the body. It creates a tissue pressure gradient that suppresses egg development. When eggs are developed artificially from stem cells, they keep on developing without pressure. When you put eggs into an incubator, the cell nuclei are rotating, and when you take them out, they stop rotating.
We also know that when an egg leaves the tissue pressure gradient in the ovary, it either ovulates and gets fertilized or dies.
This is true for all stem cells. They have to divide very slowly, or you get mutation and early cell death. Nuclei rotation in stem cells is related to the tissue pressure gradient. A greater increase in tissue pressure causes stem cells to rotate and divide more slowly, so they live longer.
Knowing this helps us understand the longevity of eggs in the ovaries and why we can limit the effects of aging with exercise, which creates myogenic tension and puts pressure on stem cells.
Now that we really understand the tissue pressure gradient and polycystic ovary syndrome better, we can delve into the science of ovarian longevity and find out why women are born with a lot of eggs or fewer eggs. Why are some lost along the way? If eggs are going to be lost by going through meiosis, without the pressure of the ovarian cortex to prevent loss, how can we make sure that migration of specified germ cells really gets into the stiff cortex, where they will be saved?
As a woman’s ovarian reserve, or egg supply, declines with age, follicular recruitment for ovulation goes down, leading to menopause. Today, we can take out an ovary, freeze it and transplant pieces of that ovarian tissue back into the woman’s body at 50 years to delay menopause and give her 20 years more function. If we keep putting tissue back every 10 years – at 60, 70 and beyond – she never has to go into menopause.
For women who don’t want to get pregnant, we place the tissue under the skin of the arm. For women who want a baby, we transplant the tissue back onto the ovary, so ovulation can resume.
There will be people who say that’s immoral, but there is no reason to accept menopause. Menopause is terrible.
It makes women’s bones weak, makes the vagina dry, increases heart disease risk and causes all kinds of other discomforts. There is no need to go through that when it can be easily avoided.
I have found that my scientist colleagues’ perceptions and resistance are more difficult to deal with than public perceptions. I can explain to the public what I’m doing, but when I do that with fellow scientists, they feel left behind. I have to explain to them how a new approach can enhance their own progress.
Professionals in this field become rigid because their whole career depends on continuing to follow the direction they are already taking, so they don’t keep an eye open to new directions. They see new approaches as threatening, rather than exciting.
The key to progress is curiosity. Curiosity is what leads to someone getting a Nobel. It’s the soul of genius.
All people are created equal, and the difference in those who bring the world forward is curiosity. It starts with how parents raise kids in their first two or three years of life. All children are born curious. If you babyproof the house and let a child explore safely, you don’t have to always say no, so you don’t turn off their curiosity. Saying yes to curiosity is where progress comes from.
This comes back to the question of ethics. Is there a serious risk to patient safety? Is there a risk to society? Do the risks outweigh the benefits?
Reproductive medicine is largely unregulated. In that void, fertility specialists and scientists are self-regulating. The people working in this field take the responsibility of ethics and safety very seriously.
Nobody is out here making “designer babies” on demand.
What we are doing is helping people have babies when they otherwise wouldn’t be able to. That’s the message I give to my patients.



