Multiplexing multiple cell lines in a single well streamlines high-content screening and reduces reagent use. Yet, accurately identifying and tracking each cell population can be complex. Reliable barcode decoding and image analysis are key to realizing its potential.
This technical note explores how microcarrier-based multiplexing enhances throughput and precision in phenotypic assays whilst maintaining data quality.
Download this application note to discover:
- How cell multiplexing can accelerate high-content screening workflows
- Techniques to identify and deconvolute populations within pooled assays
- Methods to ensure accurate clustering and phenotypic profiling across cell lines
TECHNICAL NOTE
Cell multiplexing on Opera Phenix Plus high-content screening system using SemaCyte® microcarriers.
Introduction
Cell multiplexing, the simultaneous analysis of multiple cell lines within a single well, can significantly enhance throughput while minimizing experimental time and reagent consumption. The SemaCyte® microcarrier system, developed by Semarion, provides cell multiplexing capabilities for drug discovery workflows. SemaCyte® microcarriers are microscopic, optically barcoded, flat surfaces that carry adherent cells in suspension. Cells on these carriers can be cryopreserved, pooled, dispensed, and magnetically settled to the bottom of standard microplate wells. Barcodes are encoded as dot-and-stripe patterns on each carrier’s rim and are visible in brightfield.
By leveraging the Opera Phenix Plus high-content screening system and a dedicated assay-specific building block in Harmony image analysis software, the SemaCyte® microcarriers can be detected and their optical barcodes identified to deconvolute different cell lines.
Using the cell painting assay and phenotypic profiling, this study shows that microcarriers from multiplexed wells cluster with the correct cell lines, demonstrating successful multiplexing of four cell lines per well.
Key features
• Cell line multiplexing using Semarion SemaCyte® microcarriers
• Cell Painting with PhenoVue™ Cell Painting JUMP kit
• Imaging using the Opera PhenixTM Plus HCS system
• Dedicated Find Microcarriers building block in Harmony image analysis software to deconvolute barcodes and cell lines per well
Using the cell painting assay and phenotypic profiling, this study shows that microcarriers from multiplexed wells cluster with the correct cell lines, demonstrating successful multiplexing of four cell lines per well.
Key features
• Cell line multiplexing using Semarion SemaCyte® microcarriers
• Cell Painting with PhenoVue™ Cell Painting JUMP kit
• Imaging using the Opera PhenixTM Plus HCS system
• Dedicated Find Microcarriers building block in Harmony image analysis software to deconvolute barcodes and cell lines per well
2 Multiplexing workflow
As described in figure 1, SemaCyte® microcarriers are loaded with cells (5 to 30 per carrier) by seeding four adherent cell lines (HeLa, HEK293, A549, U2OS) into four different SemaCyte® seeding dishes using standard cell culture protocols. Each 20 sq. cm dish contains 50,000 immobilized and arrayed microcarriers with the same barcode. Once cells reach optimal confluency, the SemaCytes® are released from the dish by gentle agitation, pooled, and undergo cell painting using the PhenoVue cell painting JUMP kit staining procedure. The stained microcarriers are then dispensed into a 384-well PhenoPlateTM using standard lab equipment. A magnetic plate holder ensures that the carriers are oriented correctly, with the cells facing the objective lens. Alternatively, multiplexed carriers can be dispensed into microplates and treated with drugs before cell painting. An Opera Phenix high-content screening system is used to acquire cell painting channels as well as a brightfield image for barcode identification. The dedicated building block Find Microcarriers in Harmony image analysis software deconvolutes barcodes and allows cell types to be matched to microcarriers. In this study, four cell lines were multiplexed in a single well. Further analysis steps involve a Calculate Cell Painting Properties building block and secondary dimensionality reduction by principal component analysis (PCA).
3 Image acquisition and analysis
Images of single and a 4-plexed population of microcarriers in a 384-well PhenoPlate were acquired confocally on the Opera Phenix Plus system, using a 20x high NA objective and a stack of 11 planes (figure 2). HeLa HEK293 A549 U2OS 140 μm 100 μm
Figure 2: Images of four different cell lines seeded onto different SemaCyte® barcoded microcarriers, labeled with the PhenoVue cell painting JUMP kit. Imaging was performed on an Opera Phenix Plus system using a 20xhNA objective in confocal mode. Images show microcarriers in brightfield (left) and fluorescent cell painting images (right, maximum intensity projection of 11 planes, with a plane distance of 1 μm). The Cell Painting assay stains cell organelles with 6 dyes: PhenoVue Hoechst 33342 (DNA, nucleus), PhenoVue Fluor 488-concanavalin A (endoplasmic reticulum), PhenoVue 512 nucleic acid stain (RNA and nucleoli), PhenoVue Fluor 555-WGA (Golgi and plasma membrane), PhenoVue Fluor 568-phalloidin (F-actin) and PhenoVue 641 mitochondrial stain (mitochondria).
Figure 2: Images of four different cell lines seeded onto different SemaCyte® barcoded microcarriers, labeled with the PhenoVue cell painting
JUMP kit. Imaging was performed on an Opera Phenix Plus system using a 20xhNA objective in confocal mode. Images show microcarriers in
brightfield (left) and fluorescent cell painting images (right, maximum intensity projection of 11 planes, with a plane distance of 1 μm). The Cell
Painting assay stains cell organelles with 6 dyes: PhenoVue Hoechst 33342 (DNA, nucleus), PhenoVue Fluor 488-concanavalin A (endoplasmic
reticulum), PhenoVue 512 nucleic acid stain (RNA and nucleoli), PhenoVue Fluor 555-WGA (Golgi and plasma membrane), PhenoVue Fluor
568-phalloidin (F-actin) and PhenoVue 641 mitochondrial stain (mitochondria).
To deconvolute barcodes and cell lines per well, a dedicated assay-specific building block (ABB) called Find Microcarriers was developed for Harmony™ high-content imaging and analysis software. Figure 3 shows an example of an automatic selection of unobscured microcarriers. The building block enables adjustable segmentation criteria, ranging from stringent to more permissive parameters. It also extracts barcode identifiers (currently, 16 different barcodes are available) and generates a mask for the cell-populated inner region of each microcarrier.
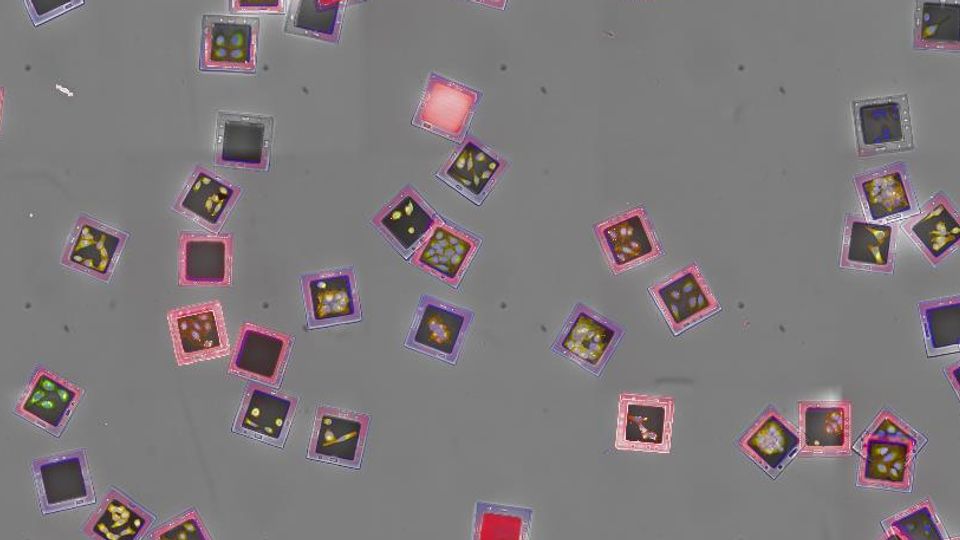
Figure 3: Harmony software with Find Microcarriers building block displaying the automatic identification of fully visible microcarriers. Flipped, overlapping or border-touching microcarriers are not selected. Image shows a global image of 25 fields of view in a maximum intensity projection of 11 planes with 1 μm distance, acquired with a 20xhNA objective.
Cell multiplexing on Opera Phenix Plus high-content screening system using SemaCyte® microcarriers.
Global Image (20x)Find MicrocarriersIdentify BarcodesMicrocarriers OKAB
Figure 4: How to use the FindMicrocarriers building block:
A:FindMicrocarriers segmentation steps: Microcarriers are segmented on a global image of 25 FOVs, barcodes are identified and carriers with readable barcodes are classified “OK”.
B: Gallery views showing microcarriers found within one well and sorted according to barcode. Red squared microcarriers are disregarded and green ones are validated for further analysis. Gallery views ease the adjustment of segmentation parameters.
Microcarrier segmentation and barcode identification are performed on a global image comprising 25 fields of view (figure 4A). Barcode identification is conducted on brightfield images. Additional gallery views facilitate the overview of selected and deselected microcarriers (figure 4B)and can be used to fine tune segmentation parameters.
Microcarriers classified as “OK” are then processed to extract 5,930 phenotypic cell painting features per cell. To associate barcodes with their respective wells, single-cell results are exported for secondary analysis (Principal Component Analysis) into Signals One™ software.
Results and discussion
The assay specific building block Find Microcarriers segments the microcarriers, reads out the barcodes and scores them as “OK” if they are readable or discards them. With the current segmentation settings, about 61% of all microcarriers are ready to undergo further analysis steps (figure 5). This percentage is the mean value of all wells and depends on the carrier´s density and the stringency of the selection criteria.
Results and discussion
The assay specific building block Find Microcarriers segments the microcarriers, reads out t heba rcodesan dsc oresth ma “ O K”if t ey a rereadable o rd scadst m.W t th c rren s egenta ions tting ,a ou 6 1%of al m cro arersa erea yt un rgofurther a nl sis s tps(fig re5 .T isp rcen agis t h m ea v al eof a llwells a nd pn son t ec rier´s d nsity a ndth string ncy o fth sele ton cr teri.
Conclusions
Here, we show how to extend a typical high-content cell painting workflow beyond a single cell type per well using SemaCyte® barcoded microcarriers.
Semarion’s microcarriers are fully compatible with the Opera Phenix Plus and OperettaTM CLS systems, offering researchers a powerful new tool for cell analysis. This technology supports cell multiplexing, allowing for concurrent analysis of multiple cell lines within a single well, boosting data output while reducing time and resources.
To streamline the analysis process, the Harmony software includes a specialized building block called “Find Microcarriers”, compatible with the 16 barcodes available from Semarion. This building block facilitates easy microcarrier segmentation, selection, barcode identification, and cell region detection. It also provides convenient gallery views for enhanced data visualization.
Assay specific building blocks like the developed “Find Microcarriers” allow rapid adoption of new sample or assay technologies. They support new image analysis applications in collaboration with researchers or technology providers.
The integration of SemaCyte® microcarriers and high-content imaging has wide-ranging applications in drug discovery, toxicology, and cancer research. It facilitates rapid evaluation of compound efficacy across diverse genetic backgrounds or cancer cell types, and facilitates the study of genetic perturbations with increased efficiency and depth.
Authors
Semarion: Daniel Weekes, Blaise Louis, Tom Mitchell, Holly Herbert, Tarun Vemulkar, Jeroen Verheyen
Revvity: Angelika Foitzik, Olavi Ollikainen, Hartwig Preckel, Kaupo Palo, Alexander Schreiner, Karin Boettcher
More information about products discussed in