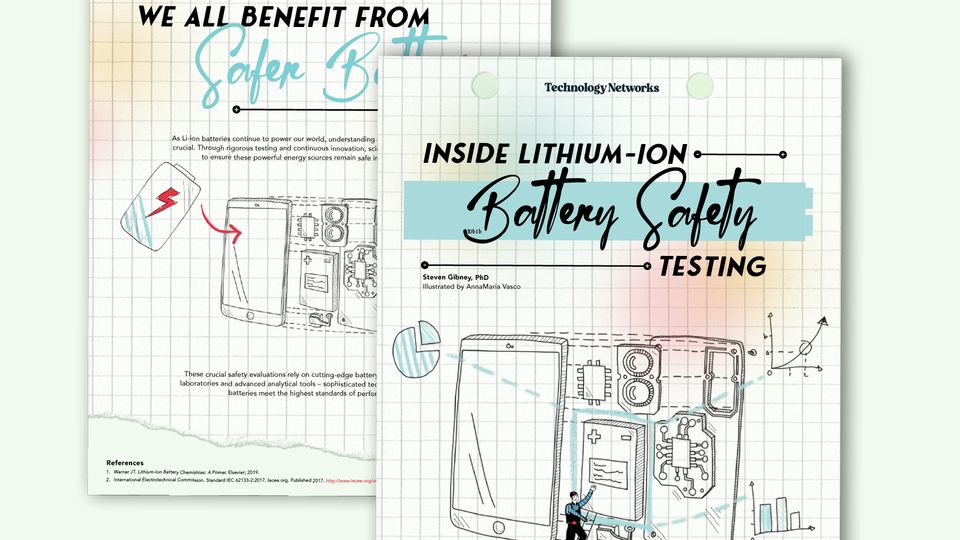
Lithium-ion batteries power our modern world from smartphones to electric vehicles, making their safety a critical concern for both manufacturers and consumers.
Despite their remarkable efficiency, these high-energy storage devices contain reactive components separated by thin barriers that can fail under certain conditions. Rigorous safety testing is essential to prevent thermal runaway, short circuits or mechanical failures.
This infographic explores the inner workings of lithium-ion batteries and the testing protocols that ensure these powerful energy sources remain safe for everyday use.
Download this infographic to explore:
- The essential components of lithium-ion batteries
- The major safety risks that can lead to battery failure
- The electrical, mechanical and thermal testing methods that ensure battery safety
Lithium-ion (Li-ion) batteries have become an everyday component of
our modern world, powering everything from smartphones to electric
vehicles. Their widespread use in daily life makes safety a priority for
manufacturers and consumers alike. This infographic explores why
battery safety is important and highlights the testing methods that
ensure the technology that powers our devices remains safe.
As Li-ion batteries continue to power our world, understanding and maintaining their safety remains
crucial. Through rigorous testing and continuous innovation, scientists and engineers work tirelessly
to ensure these powerful energy sources remain safe in our pockets and our lives.
These crucial safety evaluations rely on cutting-edge battery testing equipment, specialized
laboratories and advanced analytical tools – sophisticated technology that ensures tomorrow’s
batteries meet the highest standards of performance and safety.
References
1. Warner JT. Lithium-Ion Battery Chemistries: A Primer. Elsevier; 2019.
2. International Electrotechnical Commission. Standard IEC 62133-2:2017. Iecee.org. Published 2017. http://www.iecee.org/certification/iec-standards/iec-62133-22017
batteries
Battery Safety
testing
Inside Lithium-Ion
understanding
lithium-ion
cathode
(positive
electrode):
anode
(negative
electrode):
electrolyte:
separator:
Separator
Charging Discharging
Separator
1. Lithium ions move from the cathode to the anode
2. Electrons flow through the external circuit
3. Energy is stored in the battery’s chemical structure
Cathode
Material
Anode
Electrolyte Material
Electrolyte Electrolyte Electrolyte Electrolyte
Typically made of
lithium metal oxide,
this is responsible for
storing and releasing
lithium ions.
1. When powering a device, lithium ions move
back from the anode to the cathode
2. Electrons flow through the device, providing
electrical power
3. Energy is released as ions travel through the
electrolyte
Usually composed
of graphite, this lets
electric current flow
into the battery from
an external circuit.
A liquid or gel-like medium that allows
lithium ions to travel between electrodes.
A thin membrane between the electrodes which prevents direct
electrical contact and only allows lithium ions to pass through.
Electrolyte
Li+
Li+
e- ee-
e- e- ee-
e- e- ee-
e-
Li+
Li+
Li+ Li+
Separator
battery Safety Risks
The key components of Li-ion batteries are highly reactive and are only separated by thin barriers,
all of which are contained within a sealed unit. When these systems operate as designed, they’re
remarkably safe and efficient. However, if any component fails, the stored energy can be released in
uncontrolled and unexpected ways.
Thanks to their high energy density, good storage characteristics and long cycle life, Li-ion batteries
are essential for our modern lifestyle, powering a diversity of devices, from electric vehicles to
watches, laptops and smartphones. All of this power relies on the electrochemical behavior of the
lithium metal ions contained within the battery.1
Short circuits Thermal runaway
Thermal runaway occurs
when a battery’s internal
temperature spirals out of
control. This begins when
temperatures rise beyond
the battery’s designed
safe operating range,
creating a self-perpetuating
cycle, potentially leading
to smoke, fire or even
explosive conditions.
Short circuits are created when
an unintended electrical path
forms between the battery’s
positive and negative terminals.
This leads to an uncontrolled
discharge of electrical energy
that generates extreme heat.
Mechanical
damage
When a Li-ion
battery is charged
beyond its
designed voltage
limit, its internal
chemistry begins
to break down.
This can lead to
short circuits, reduced capacity and
increased risk of thermal events.
Overcharging
Any physical impact, compression
or puncture can compromise the
separator between electrodes.
Even minor damage can create
internal shorts, disrupt ion flow
or create points of weakness that
may lead to battery failure.
how do you
knowb aattery is safe? Rigorous testing protocols ensure batteries meet international safety standards.2 Each battery design
undergoes multiple testing phases before approval. These tests simulate real-world conditions and
potential abuse situations, helping manufacturers identify potential failure modes and validate safety
features before batteries reach consumers.
ensures that batteries
operate safely within
their specified voltage
and current ranges
and that protection
mechanisms function
correctly when these
limits are exceeded.
prevents batteries from
being damaged by charging
them too much.
testing determines how
an electrical system
responds to short circuit.
testing confirms that the
battery does not malfunction
or become damaged under
voltage fluctuations.
electrical
testing
Overcharge
protection
Short circuit
response
Voltage
tolerance
simulates various
real-world scenarios
where batteries might
experience physical
trauma or pressure.
evaluates how
batteries respond to
temperature changes
and thermal stress.
under controlled pressure.
tests the battery
at sustained high
temperature conditions.
from various heights
and angles.
involves repeated heating
and cooling between
−20 °C and +60 °C.
evaluation determines
the strength the film that
separates the electrodes.
ensures internal chemical
changes are as expected at
a range of temperatures.
Mechanical
testing
Thermal
testing
Crush
testing
Heat
exposure
Drop testing
Temperature
cycling
Puncture
resistance
Thermal stability
monitoring
we all benefit from Safer Batteries
Steven Gibney, PhD
Illustrated by AnnaMaria Vasco